Epigenomics, the study of chemical modifications in DNA and associated histone proteins, plays a crucial role in understanding human diseases, including cancer. These modifications significantly impact cancer formation, progression, and treatment response. At Johns Hopkins University, the Epigenome Sciences Cluster is at the forefront of this research, bridging the gap between laboratory discoveries and clinical applications to accelerate the development of innovative treatments.
Pediatric brain tumors represent one of the most challenging areas in oncology. Standard treatments typically involve surgery, followed by radiation and chemotherapy. However, predicting which patients will respond to these treatments and which will encounter resistant tumors remains difficult. This challenge highlights the need for a deeper understanding of the role of epigenetics in treatment response, which could lead to personalized therapies and novel molecular targets.
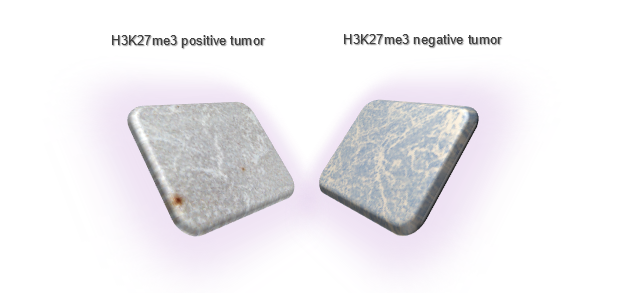
A critical discovery in this field is the loss of an epigenetic mark known as histone H3 tri-methylation (H3K27me3), a feature common to several aggressive pediatric brain tumors, including Posterior Fossa A (PFA) ependymoma and midline glioma. In a recent editorial, our Epigenome Sciences Cluster Investigator, Dr. Michael Goldstein, explores the significance of this epigenomic alteration and its impact on treatment outcomes (Goldstein, Oncotarget 2023). Notably, the Goldstein lab has uncovered that H3K27me3 loss is also prevalent in Group 3 medulloblastoma, another pediatric brain tumor (Gabriel N et al., Cancer Research, 2022). This loss triggers a signaling cascade that renders medulloblastoma cells resistant to radiation, leading to recurrence and poor survival rates. Building on these findings, they have developed a targeted therapeutic approach to counteract radiation resistance and enhance the efficacy of radiotherapy in these tumors. The lab is now evaluating several agents in cell and animal models to identify the most promising candidate for clinical use in pediatric patients.
Whether H3K27me3 loss contributes to radiation resistance in PFA ependymoma and midline glioma remains an open question. Given the difficulty in treating these tumors, improving radiation effectiveness could significantly benefit patient outcomes. Based on their work with medulloblastoma, Dr. Goldstein hypothesizes that targeting this unique epigenetic trait may also overcome treatment resistance in these tumors. He will leverage the collaborative environment fostered by the Epigenome Sciences Cluster to advance this research, paving the way for personalized, epigenetically-guided treatments for aggressive pediatric brain cancers.

